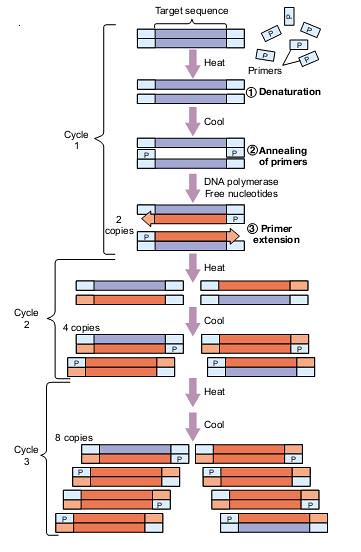
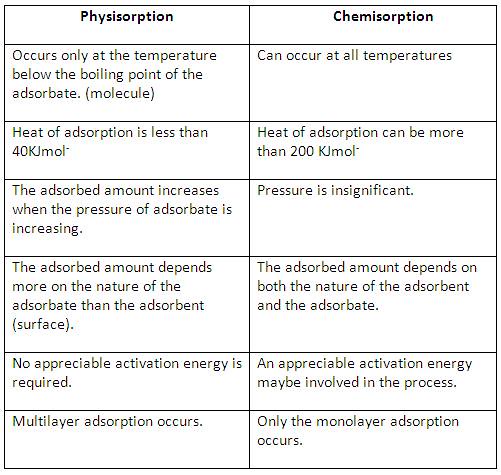
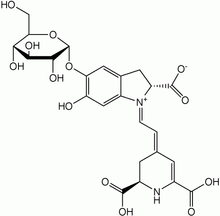
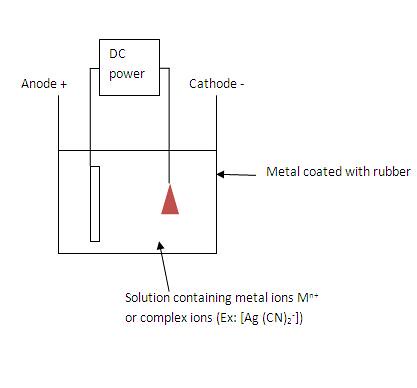
Phytophthora infestans
Obligate parasite.
Infect potatoes and
tomatoes with late blight disease.
Life cycle
Mycelium: Non septate, coenocytic
Asexual
reproduction
In the host tissue inter-cellular
somatic hyphae colonizes and club shaped haustorias penetrate the tissues,
feeding the parasite.
Sporangiophores are
produced from the hyphae and emerge through stomata.
Sporangiophores
produce lemon-shaped sporangia laterally and at the terminal end/apex of the
sporangiphore. A Sporangium possesses a papilla on its tip.
Outcome of the
sporangium depends on the temperature and humidity.
Low
humidity or high temperature:
Sporangium germinates by a germ tube that later results the somatic
hapha.
High
humidity (when raining) or low temperature (120C or below: Cytoplasm of the sporangium divides and gives
rise to biflagellate Zoospores. Either by dissolution of papilla or
through its opening tip, zoospores are released to outside. Zoospores directly
penetrate host tissue and undergo encystment in cells. They develop into germ
tubes that later result the hyphae.
Sexual
reproduction
Plasmogamy occurs as
oogonium punches the antheridium, going through that antheridium, oogonium
grows above it. The antheridium therefore appears as a funnel at the base
around the oogonium. Karyogamy and meiosis occurs respectively. Oospore is
spherical and adapted to adverse environmental conditions. It results out a
sporangiophore that again develops into a sporangium.
 |
| via www.apsnet.org |
Plasmopara viticola
Obligate parasite.
Infect grape vines with Downy mildews.
Life Cycle
Mycelium is non septate and coenocytic.
Life cycle is much similar to Phytophthora
infestans as both are oomycites.
Asexual reproduction
Somatic hyphae develops in inter cellular space host
tissue penetrating with haustoria.
Hyphae give rise to sporangiophores that emerge
through stomata, branching and producing sporangia at each apex of the
branches.
Sporangia may either germinate directly into mycelium
or produce kidney shaped, biflagellate zoospores, depending on humidity and
temperature just like the Phytophthora infestans. Zoospores encyst in
cells, germinate and result the somatic hyphae.
Sexual reproduction
Sexual phase is taken place at unfavorable conditions.
Plasmogamy occurs by gametangial copulation as oogonium and anthredium come
close together. Karyogamy results zygote that later gives the spherical
oospore. Oospore divides by meiosis and undergoes germination producing a
sporangium.
More about downy mildew http://fruit.cfans.umn.edu/grape/IPM/downy.pdf