Catechol
Resorcinol
2-napthol
Solubility
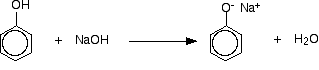
Some phenols (Ex: Phenol,catechol,resorcinol) are soluble in water and others are sparingly soluble or insoluble in water because of the increasing molecular weight and hydrophobic nature. But all the phenols are capable of reacting with NaOH solution and dissolve.
C6H5OH + NaOH ----> C6H5ONa + H2O
Reaction with Sodium carbonate
Phenols give no reaction with sodium carbonate but as the aqueous solution of it was used,phenols dissolved in it giving the same colourations given when testing the solubility in water.As Phenols do not produce gas from sodium carbonate it should be a very weak acid.
Benzoylation
To react with benzoyl chloride, phenol should be modified to make the reaction possible.the -COCl group is bounded directly to the benzene ring. Therefore it's less reactive.phenol is first dissolved in NaOH in order to get the phenoxide. Later it's reacted with the Benzoyl chloride.





2. Why is Na2CO3 added to phenols to keep them in the aqueous phase. Justify your answer with a chemical equation
ReplyDelete