Electroplating is the
process of depositing a layer of metal electrolytically on to a surface.The
articles to be plated make the cathode of an electrolytic cell and a rod of the
plating metal makes the anode.
Normally pure metals
are used. E.g. Cu, Ni, Cr, Au, Ag, Pt, Zn
But there are exceptions
such as Alloys (Cu-Zn, Ni-Cr) and metal with polymers or ceramics (metal-PTEF,
metal- Ceramic)
Requirements for
electroplating
-Proper bonding
between the plating material and the surface.
-Evenness of the
plating.
-Cleanliness
-Leak holes must not
be left.
-Texture: should have
high brightness.
-Resistant to
chemicals in the environment that can cause damage.
Essential parts
of electroplating
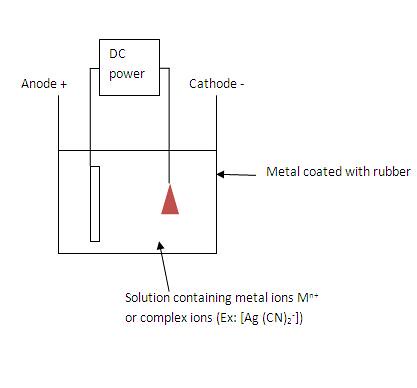
Difference between
Mn+ and complex ions
Complex ions release
metal ions slowly. Therefore its concentration is low. It’s important to obtain
a smooth ending.
Additional electrolyte
can be used to increase the conductivity of the system but it will not effect
the solution’s ions. Sodium sulphate is an additional electrolyte.
Additives
Additives increase the
quality of the electroplating.
-Brighteners: Saccaric
acid, Thiourea
-Levelers: Formaldehydes
-stress relievers: organic
substances
-wetting agents:
Sodium lauryl suphate
Factors affecting
the quality of the electroplating
-Nature of the
electrolyte
-Concentration of the
electrolyte
-Purity of the
electrolyte
-Nature of the
additives
-Concentration of the
additives (should be low)
-pH of the solution
-Temperature
-Current density
-Geometry of the
electrode(whether it’s round or flat)
-Shape of the bath
-Flow conditions
(stirring is good)
Hull cell
To study the quality of electroplating dependence on the current density.
Haring-Blum cell
To determine the
throwing power of the electroplating process.
Your content helped me a lot to take my doubts, thank you very much.
ReplyDelete